Ο αθλητισμός που ενώνει…

Ο Βασίλης Σπανούλης πήγε προς το μέρος όπου βρισκόταν η παλιά «φρουρά» της Εθνικής και χάθηκε στις αγκαλιές των Θοδωρή Παπαλουκά, Δημήτρη Διαμαντίδη και Κώστα Τσαρτσαρή.

RCTs φάσης 3 με ταυτόχρονη δημοσίευση
ΑΝΤΙΥΠΕΡΤΑΣΙΚΑ: Η αντιμετώπιση της ανθεκτικής και μη ελεγχόμενης υπέρτασης αποτελούν τις κύριες προκλήσεις στα υπερτασικά ιατρεία
BaxHTN trial;a potential game changer’ for treatment-resistant hypertension
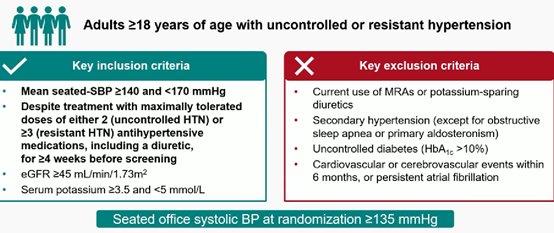
Baxdrostat (an aldosterone synthase inhibitor) led to substantial BP lowering, with 40% of patients with uncontrolled hypertension reaching BP of less than 130 mm Hg. Treatment was well-tolerated
Flack JM, Azizi M, Brown JM, et al. Efficacy and safety of baxdrostat in uncontrolled and resistant hypertension. N Engl J Med. 2025;Epub ahead of print.
Εditorial. Guzik TJ, Tomaszewski M. Aldosterone synthase inhibition for hypertension. N Engl J Med. 2025;Epub ahead of print.
RE TREAT FRAIL trial: μια μελέτη που προσπάθησε να δείξει όφελος με μείωση (step down) των αντιυπερτασικών φαρμάκων
Compared with usual care, stepping down antihypertensive therapy in nursing home patients aged 80 years and older did not yield any reduction in major adverse events or correlate with any other clinical benefit
A Benetos et all. Reduction of Antihypertensive Treatment in Nursing Home Residents. N Eng J Med 2025 in press
KAΡΔΙΑΚΗ ΑΝΕΠΑΡΚΕΙΑ
DAPA ACT HF – TIMI 68 trial : H ενδονοσοκομειακή έναρξη SLGT2i σε Οξεία ΚΑ δεν έπιασε το καταληκτικό σημείο αλλά ή συνολική εικόνα υποστηρίζει την πρώιμη τους έναρξη.
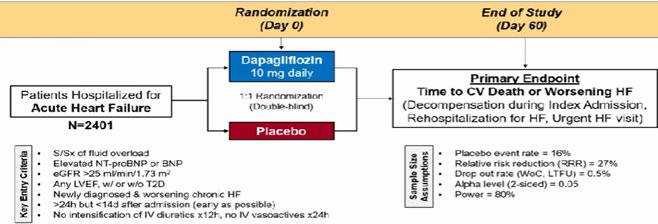
In a prespecified meta-analysis of three trials evaluating in-hospital initiation of SGLT2i) in patients hospitalized for HF, SGLT2i appeared to reduce the early risk of cardiovascular death or worsening HF (HR 0.71; 95% CI 0.54-0.93) and of all-cause death (HR 0.57; 95% CI 0.41-0.80).
David D. Berg, Siddharth M. Patel, Paul M. Haller et al. Dapagliflozin in Patients Hospitalized for Heart Failure: Primary Results of the DAPA ACT HF-TIMI 68 Randomized Clinical Trial and Meta-Analysis of SGLT2i in Patients Hospitalized for Heart Failure. Circulation 2025 in press
VICTOR trial: Vericiguat failed to provide an overall benefit for patients with HFrEF but without signs or symptoms of clinical worsening over roughly 18 months of follow-up
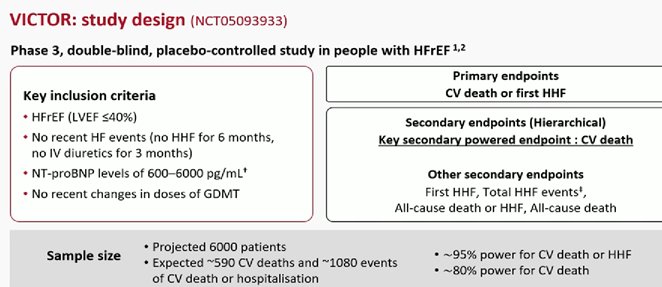
randomly assigned 6,105 patients with HFrEF but without a HF hospitalization in the prior 6 months or outpatient IV diuretic use within the prior 3 months (median age, 68 years; 23.6% women; 64.4% white) to receive vericiguat or placebo.
VICTOR/VICTORIA meta-analysis of patients with chronic or worsening HFrEF, vericiguat showed consistent benefit
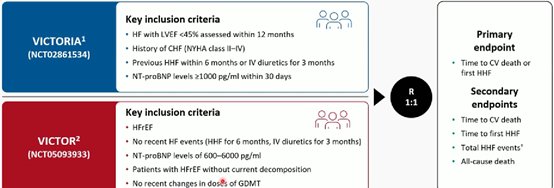
The prespecified patient-level meta-analysis of VICTOR and VICTORIA included 11,155 patients (mean age, 68 years; 23.7% women; 64.3% white). Median follow-up was 15.6 months and the rate of ascertainment was 99.4%.
Το όφελος παρατηρήθηκε σε NT pro BNP < 6000pg/ml κατι σύνηθες στη καθη μερα πράξη σ ασθενείς με HFrEF(ανεξήγητο )
HFrEF pharmacology meta-analysis
In a meta-analysis of 103,754 patients with HFrEF from 89 randomized controlled trials, quintuple therapy including an angiotensin receptor/neprilysin inhibitor, beta-blocker, mineralocorticoid receptor antagonist, SGLT2 inhibitor and vericiguat reduced mortality risk by the greatest degree compared with no therapy (HR = 0.3; 95% CI, 0.22-0.39), followed by quadruple therapy with an angiotensin receptor/neprilysin inhibitor, beta-blocker, mineralocorticoid receptor antagonist and SGLT2 inhibitor (HR = 0.34; 95% CI, 0.22-0.44)
Butler J, McMullan CJ, Anstrom KJ, et al. Vericiguat in patients with chronic heart failure and reduced ejection fraction (VICTOR): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2025;Epub ahead of print.
Javed Butler, Francesco Fioretti , Ciaran J. McMullan et al. Vericiguat and mortality in heart failure and reduced ejection fraction: the VICTOR trial Εur Heart J 2025 in press
Zannad F, O’Connor CM, Butler J, et al. Vericiguat for patients with heart failure with reduced ejection fraction across the risk spectrum: an individual participant data analysis of the VICTORIA and VICTOR trials. Lancet. 2025;Epub ahead of print.
Zannad F, Reddy YNV, Barash I, et al. Effect of vericiguat on worsening heart failure in compensated outpatients with HFrEF: insights from VICTOR. JACC. 2025;Epub ahead of print
Bart J. van Essen, Daan C.H. Ceelen, Wouter Ourworker. Pharmacological Treatment of Heart Failure with Reduced Ejection Fraction: An Updated Systematic Review and Network Meta-Analysis JACC 2025 inpress
DIGIT-HF: COME BACK of Digitoxin in Patients With HFrEF
The AHA guidelines on the treatment of heart failure state glycoside agents can be considered for patients with HFrEF who remain symptomatic despite guideline-directed medical therapy or who cannot tolerate such treatment, with the aim of reducing hospitalizations for heart failure
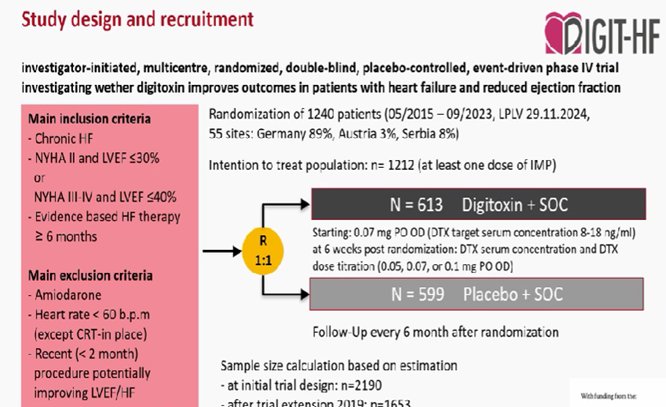
Η διγιτοξίνη και όχι η διγοξίνη που σ DECISION προσεχής μελέτη μείωσε το σύνθετο καταληκτικό σημείο ολικής θνητότητα ,επανανοσηλειών μεταξύ ασθενών με προχωρημένη (HFrEF). Η μελέτη χρειάσθηκε 8 χρόνια για περισυλλογή ασθενών και δεν παρατηρήθηκε διαφορά στον αιφνίδιο θάνατο.
many patients in DIGIT-HF were not receiving all of the “fantastic four” therapies at baseline(40% ARNI, 70% MRA, 64% had a ICD, and 25% had received CRT), “For these reasons, the DIGIT-HF trial may not be definitive enough to influence guideline recommendations without confirmation from additional clinical trials
CTA
DANCAVAS II trial
Invitation to a comprehensive CT-based screening for subclinical CVD did not decrease death 20 over 7 years among men aged 60–64 years but did increase severe bleeding. Because the trial was 21 powered for events over 10 years, further follow-up is needed
Jes S. Lindholt et al. Outcomes of cardiovascular screening in men aged 60–64 years: the DANCAVAS II trial 5. Circulation 2025 in press
PULSE trial: No Benefit to Routine CT Angio After Left Main PCIEuropean guidelines give a weak class IIb recommendation for surveillance stress testing after PCI, but the US guidelines for managing patients with ACS do not make any such provisions. For patients undergoing PCI for chronic coronary syndromes, the US guidelines advise against routine periodic testing with coronary CT angiography or stress testing if there is no change in clinical or functional status.
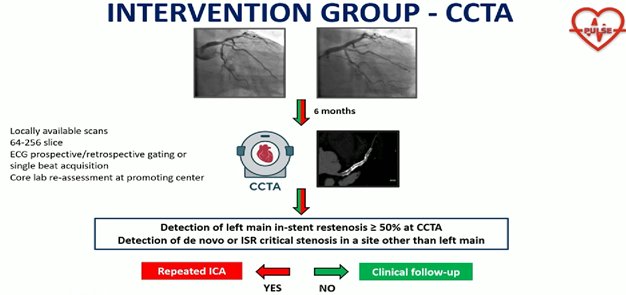
fail to find a benefit from regular checks after PCI, 6 μήνες this time in a higher-risk population. Routine CCTA after LM PCI did not reduce the composite primary endpoint, but was associated with fewer spontaneous MIs and more imaging-triggered revascularizations.
D’Ascenzo F, Cerrato E, De Filippo O, et al. Computed tomography angiography or standard care after left main PCI. JACC. 2025: Epub ahead of print.
ΚΟΛΠΙΚΗ ΜΑΡΜΑΡΥΓΗAMALFI trial: Remote Screening Using ECG Patch Demonstrated Modest Benefits in AFib Diagnosis
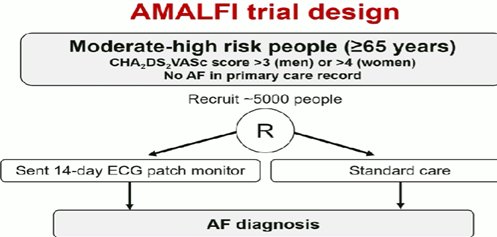
H χρήση ΗΚΓ patch για από μακριά παρακολούθηση(screening) ηλικιωμένων ασθενών μετρίου – αυξημένου κίνδυνου για ΑΕΕ έδειξε μέτρια οφέλη στη μακροχρόνια διάγνωση ΚΜ αλλά και έκθεσης σε αντιπηκτικά. JAMA 2025 in press
ALONE AF trial: Η μελέτη υποστηρίζει ότι η διακοπή αντιπηκτικών μετα ablation αντιπηκτικών μπορεί να ωφελήσει ειδικά του αυξημένου κινδύνου ασθενείς. Για εκείνους με CHA2DS2-VASc score >3 or prior stroke), δεν το συζητάμε συνεχίζουν μεχρι νεωτέρας . The mean CHA2DS2-VASc score was 2.1 and 67.6% of the cohort underwent ablation for paroxysmal AF
Gerstenfeld EP, et al. JAMA. 2025;doi:10.1001/jama.2025.14669.
Kim D, et al. JAMA. 2025;doi:10.1001/jama.2025.14679
ABC AF trial: risc score βασισμένο σε βιοδείκτες
The individually tailored multidimensional treatment strategy, based on ABC AF risk scores, did not improve clinical outcomes as compared with usual guideline-based care in patients with AF. Circulation 2025 in press
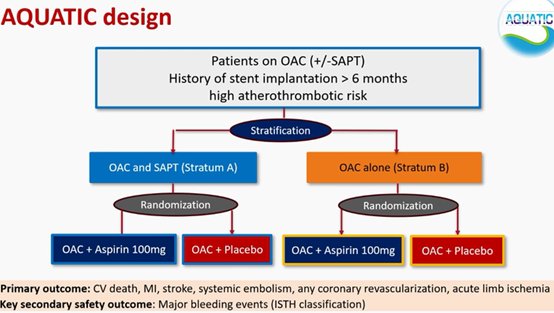
AQUATIC trial: Ασθενείς με ΚΜ ακόμη και αυξημένου θρομβοεμβολικού κινδύνου με stent για τουλ 6 μήνες δεν χρειάζεται να παίρνουν μαζί με το αντιπηκτικό και ασπιρίνη. Η μελέτη σταμάτησε πρόωρα στα 2.2 χρόνια λόγω αυξημένης θνητότητας
ΛΙΠΙΔΙΑ – ΤΡΙΓΛΥΚΕΡΙΔΙΑ
VICTORION trial: Inclisiran, a small interfering ribonucleic acid (siRNA) that targets hepatic proprotein convertase subtilisin/kexin type 9 (PCSK9) messenger RNA
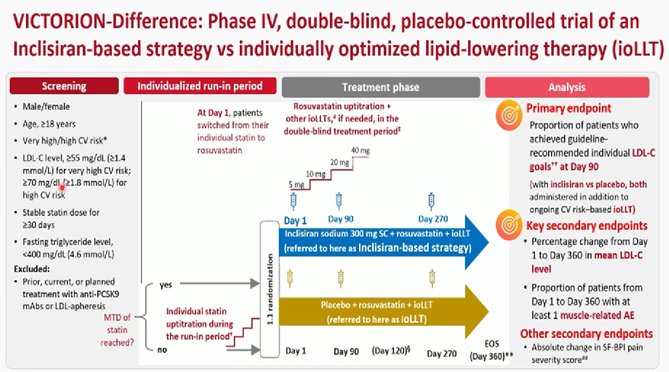
An inclisiran-based treatment strategy was superior to ioLLT in LDL-C goal achievement, delivering early and sustained LDLC reduction, with fewer muscle-related adverse event MRAEs in individuals with hypercholesterolaemia
Ulf Landmesser, Ulrich Laufs , Ulrike Schatz et al. Inclisiran-based treatment strategy in hypercholesterolaemia: the VICTORION-difference trial. Eur Heart J 2025 in press
ΕSSENSE TIMI 73b trial : Olezarsen Brings Moderately High Triglycerides Under Control
Olezarsen an antisense oligonucleotide that targets the messenger RNA for apolipoprotein C-III, which is a target for triglyceride lowering.was approved by the FDA in 2024 for the treatment of familial chylomicronemia syndrome.
I the monthly injectable lowered levels by nearly 60 percentage points vs placebo, but will it impact events?
Determining whether these changes to the lipid profile would translate into a clinical benefit with respect to cardiovascular outcomes would require a dedicated trial
Bergmark BA, Marston NA, Prohaska TA, et al. Targeting APOC3 with olezarsen in moderate hypertriglyceridemia. NEJM. 2025;Epub ahead of print.
Eν το μεταξύ ο δικός μας Sam Tsimikas ανακοίνωσε τις μελέτες CORE Olezarsen reduced triglycerides and the rate of acute pancreatitis events in patients with severely elevated triglycerides που επιβεβαιωνει τα παρα πα΄νω
ΝΕWTON CABG trial
Η εντατική μείωση της LDL με evolocumab, υποδόρια κάθε 2 εβδομάδες 3-21 ημερες μετά το CABG δεν επηρέασε τη φλεβικά μοσχεύματα μετά 2 χρόνια παρακολούθηση. Το αρνητικό αποτέλεσμα ήταν αναμενόμενο γιατί στα αρχικά στάδια επικρατεί η θρόμβωση στα μοσχεύματα και η αθηροσκληρωση ακολουθεί
Verma S, Leiter LA, Teoh H, et al. Effect of evolocumab on saphenous vein graft patency after coronary artery bypass surgery (NEWTON-CABG CardioLink-5). Lancet 2025; published online
PREVENT MINS trial: Η Ivabradine σε μη καρδιακά χειρουργεία δεν μείωσε την μυοκαρδιακή βλάβη(myocardial injury after noncardiac surgery MINS
Wojciech Szczeklik, Jakub Fronczek, Zbigniew Putowsk et al Ivabradine in Patients Undergoing Noncardiac Surgery. Circulation 2025 in press
POTCAST trial: Ισως το ψηλό κάλιο ωφελεί τους ασθενείς με ICD
Would we benefit the patients by changing or increasing the plasma potassium?” In patients with an ICD and plasma potassium ≤4.3 mmol/L, boosting the level to 4.5–5.0 mmol/L reduced the risk for ventricular arrhythmias.
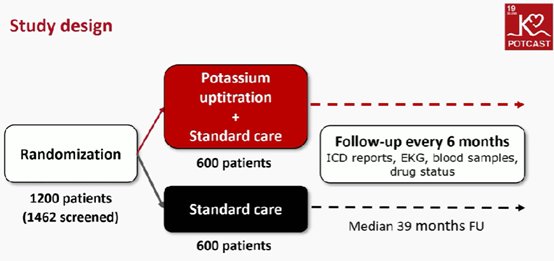
Θεραπευτική αύξηση των επιπέδων καλίου(> 4.3 μMmol/l σ ασθενείς με ICD βελτιώνει τον κίνδυνο των κοιλιακών αρρυθμιών.(mean age, 63 years; 20% women)
Those assigned to the potassium regimen received potassium supplementation, a mineralocorticoid receptor antagonist or both, plus dietary guidance with a goal of achieving serum potassium levels of 4.5 mmol/L to 5 mmol/L
Β AΠΟΚΛΕΙΣΤΕΣ μετά από ΕΜ σ ασθενείς με φυσιολογικό > 50% ή ήπια επηρεασμένο ΚΕ (40-49%)
we should continue to offer b-blockers following MI in patients with reduced LVEF, but it is reasonable to reevaluate whether to maintain beta-blockers after the dust has settled or about 4-6 weeks after the event,” me μια επιφύλαξη στις γυναίκες kai στους >75 ετών

the US guidelines for the treatment of chronic coronary syndromes, long-term use of beta-blockers is no longer recommended for patients without MI in the past year, without impaired LVEF, or without another primary indication for beta-blocker therapy, such as angina, arrhythmias, or hypertension. In patients who’ve had an MI in the past year, physicians are advised to reassess the indication for long-term use (> 1 year) for reducing MACE (class 2b recommendation) H μελέτη REBOOT-CNIC δεν έδειξε ευνοϊκά αποτελέσματα ενώ η BETAMI-DANBLOCK albeit with a small effect size in a nonfatal utcome. Επανεμφραγματα. 86% of those in the beta-blocker arm in REBOOT-CNIC received bisoprolol, and more than 90% in BETAMI-DANBLOCK received metoprolol. All patients in CAPITAL-RCT who were assigned a beta-blocker received carvedilol.
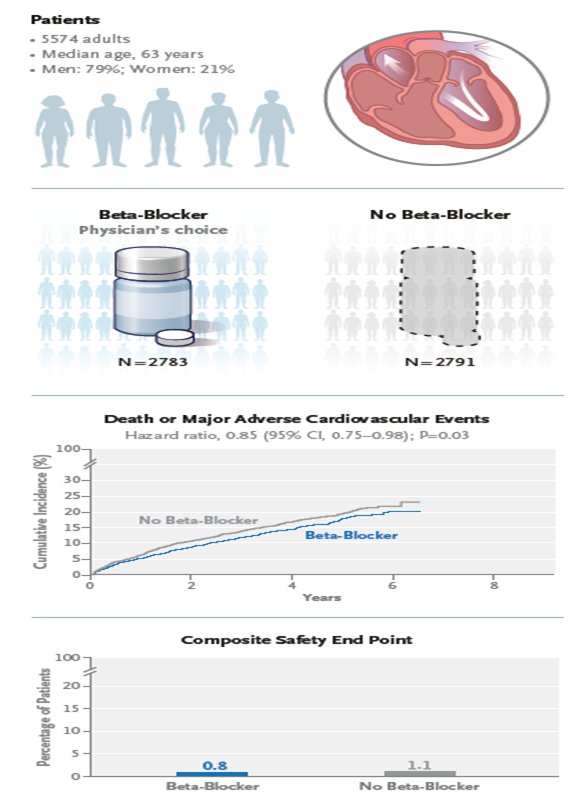
BETAMI DANBLOCK trial
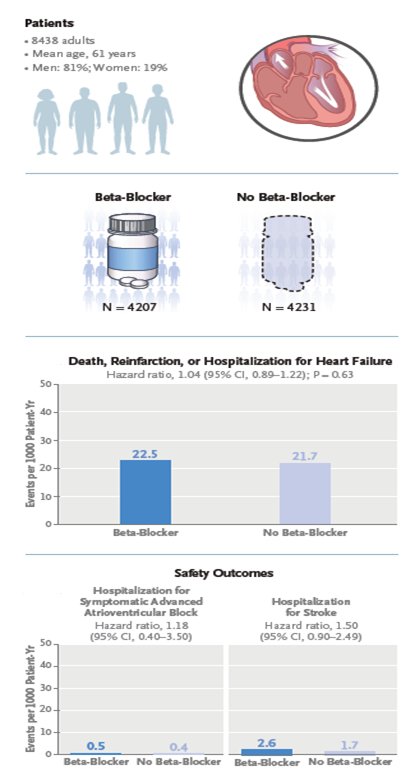
REBOOT trial
61yr 19,5% women, 11.5% LVEF <50% 51% dyslipidemia, 50% hypertension, 45% smokers 21% DM, 51% STEMI,49% NSTEMI,25% MVD, 94% PCI, 88% complete revasc
Ανάλυση υποομάδας In the REBOOT trial of MI patients managed according to contemporary standards, beta-blocker therapy was associated with evidence of harm in women—particularly those with preserved LVEF .50% and receiving higher doses—an effect not observed in men. Eur Heart J 2025 In press
Ibáñez B, Latini R, Rossello X, et al. Beta-blockers after myocardial infarction without reduced ejection. N Engl J Med. 2025;Epub ahead of print.
Munkhaugen J, Kristensen AMD, Halvorsen S, et al. Beta-blockers after myocardial infarction without heart failure. N Engl J Med. 2025;Epub ahead of print.
Rossello Lozano X, Prescott EIB, Kristensen AMD, et al. Beta-blockers after myocardial infarction with mildly reduced ejection fraction: an individual patient-data meta-analysis of randomised, controlled trials. Lancet. 2025;Epub ahead of print. Volume 406, Issue 10508p1128-1137
Roselto X et al. Beta-blockers after myocardial infarction: effects according to sex in the REBOOT trial . Eur Heart J 2025 in press
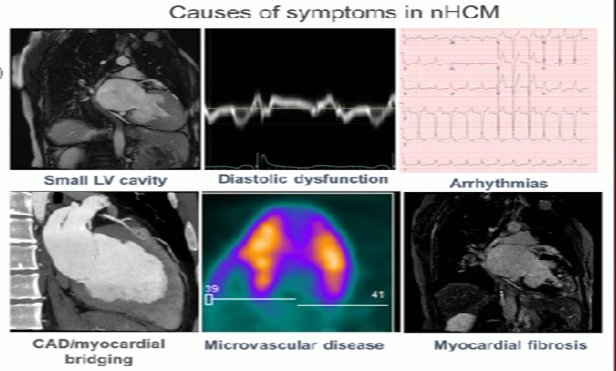
ΥΠΕΡΤΡΟΦΙΚΗ ΜΥΟΚΑΡΔΙΟΠAΘΕΙΑ: αποφρακτική και μη
Ανασκόπηση από τον πρωτοπόρο του είδους E. Braunwald N Engl J Med 2025;393:1004-1015 και δύο μελέτες στο ESC 25.
MAPLE- HCM trial: Aficamten νέος αναστολέας μυοσύνης superior to metoprolol (improved peak oxygen uptake at 24 weeks vs. in patients with obstructive hypertrophic cardiomyopathy.
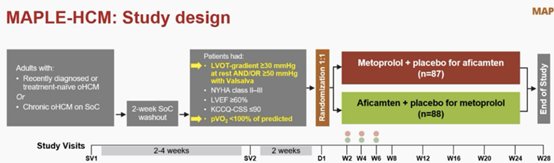
Aficamten appeared to be as safe as metoprolol in this population
ΣοC standa of care δόσεις μετοπρολολης 50-200 και αφικαμτένης 5 -20 mg
Aficamten is currently being reviewed by the US Food and Drug Administration, with a decision expected by December 26,ODYSSEY-HCM trial: Το Mavacamten δεν βελτίωσε την έκβαση σε συμπτωματικούς ασθενείς με μη αποφρακτική HOCM( nΗCM)
García Pavía P, Maron MS, Masri A, et al. Aficamten or metoprolol monotherapy for obstructive hypertrophic cardiomyopathy. N Engl J Med 2025;393:949-960
Desai MY, Owens AT, Abraham T, et al. Mavacamten in symptomatic nonobstructive hypertrophic cardiomyopathy. N Engl J Med 2025;393:961-972
Εditorial. Amrut V. Ambardekar Expanding the Role of Myosin Inhibition in Hypertrophic Cardiomyopathy — A Tale of Two Conditions N Engl J Med 2025;393:1026-1028
ANTIΘΡΟΜΒΩΤΙΚΗ ΘΕΡΑΠΕΙΑ ΜΕΤΑ ΕΠΑΝΑΓΓΕΙΩΣΗ
Post PCI NEO MINDSET trial:
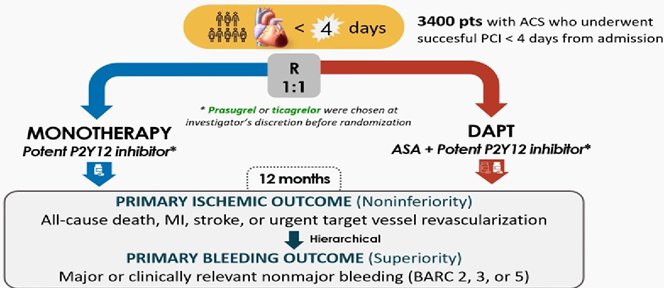
Among patients who had undergone successful PCI for acute coronary syndromes, potent P2Y12 inhibitor monotherapy was not found to be noninferior to dual antiplatelet. Aspirin is still an important drug, at least in these very early phases after infarction and PCI. Guimarães PO, et al. Early Withdrawal of Aspirin after PCI in Acute Coronary Syndromes N Engl J Med. 2025;in press
TARGET FIRSTMI trial
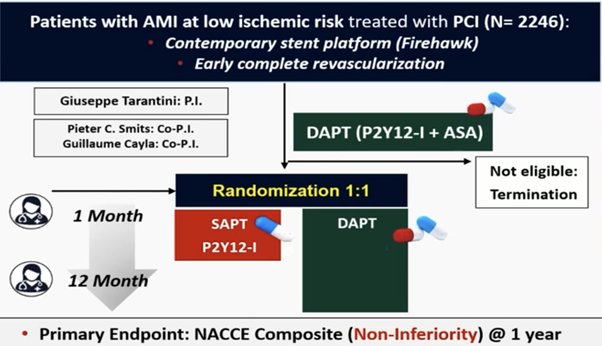
Among low-risk patients with acute myocardial infarction who had undergone early complete revascularization and had completed 1 month of dual antiplatelet therapy without complications, P2Y12-inhibitor monotherapy was noninferior to continued dual antiplatelet therapy with respect to the occurrence of adverse cardiovascular and cerebrovascular events and resulted in a lower incidence of bleeding events. therapy with respect to a composite of death or ischemic events at 12 months
Tarantini G et al. Early Discontinuation of Aspirin after PCI
in Low-Risk Acute Myocardial Infarction. Ν Engl J Med 2025 in press
TAILORED CHIP trial:
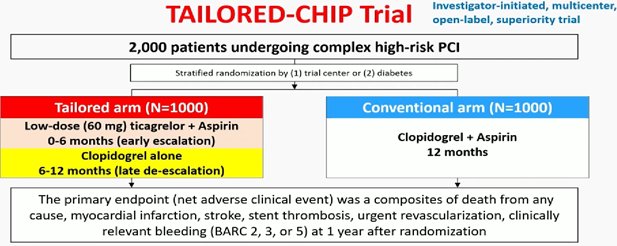
Among high-risk patients undergoing complex PCI, tailored antiplatelet strategy with early escalation and late de-escalation, as compared with dual antiplatelet therapy, did not decrease the incidence of primary net adverse events at 12 months. Eur heart J 2025
PARTHENOPE trial
In patients undergoing PCI, a personalized DAPT duration from 3 to 24 months based on a clinical risk score led to a lowered risk of net adverse clinical events than standard care consisting of 12 months of DAPT Piccolo R, Calabro P, Carrara G, et al. Personalized or Standard Duration of Dual Antiplatelet Therapy after Percutaneous Coronary
Intervention: PARTHENOPE Randomized Trial. JACC 2025; in press.
TADCLOT trial
Ticagrelor was not superior to BID(δύο φορές) clopidogrel in reducing MACE at one month after primary PCI, and bleeding rates were similar. However, event rates were lower than anticipated and ticagrelor significantly reduced MACE within the first 2 weeks compared with BID clopidogrel.
Abdul Hakeem et al. Twice-A-Day CLOpidogrel vs Ticagrelor to Reduce Short-Term MACE after Primary PCI. JACC 2025 in pressPost CABG : TACSI trial
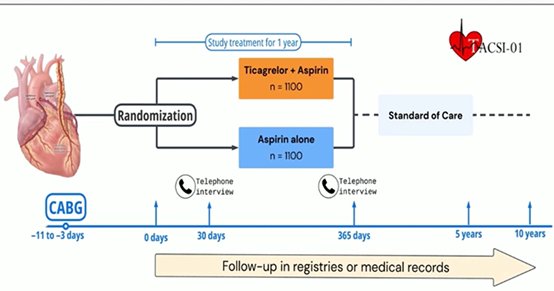
Among patients who underwent CABG for an ACS, ticagrelor plus aspirin did not result in a lower incidence of death, myocardial infarction, stroke, or repeat coronary revascularization than aspirin alone at 1 year
Ταυτόχρονα δημοσιεύτηκε Antithrombotic therapy after coronary artery bypass graft surgery a Clinical Consensus Statement of the ESC Working Group on Cardiovascular Surgery, the ESC Working Group on Cardiovascular Pharmacotherapy, and EACTS Εur Heart J 2025
ΠΛΗΡΗΣ ΕΠΑΝΑΓΓΕΙΩΣΗ: άμεσα στον ίδιο χρόνο ή προγραμματισμένα(staged). Ασιάτες ασθενείς
ΟPTION STEMI trial: μη τη συγχέεται με την OPTION που συνέκρινε τη σύγκλειση του ωτίου αρ κόλπυ με αντιπηκτικά
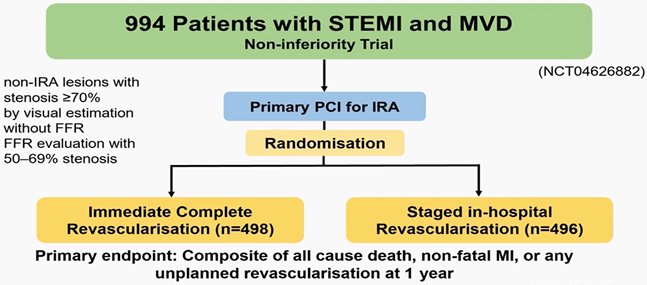

The optimal timing of complete revascularisation for patients with STEMI and multivessel coronary artery disease remains unclear. Among patients with STEMI and multivessel disease, immediate complete revascularisation
was not shown to be non-inferior to staged complete revascularisation during the index admission in terms of incidence of a composite of death from any cause, non-fatal myocardial infarction, or any unplanned revascularisation at 1 year.
Kim, MC ∙ Ahn, JH ∙ Hyun, DY ∙ et al. Immediate versus staged complete revascularisation during index admission in patients with ST-segment elevation myocardial infarction and multivessel disease (OPTION–STEMI): a multicentre, non-inferiority, open-label, randomised trial. Lancet. 2025;406:1032-1043
HI PRO trial: Patients with Provoked Venous Thromboembolism Plus Other Risk Factors: Extend Anticoagulation?
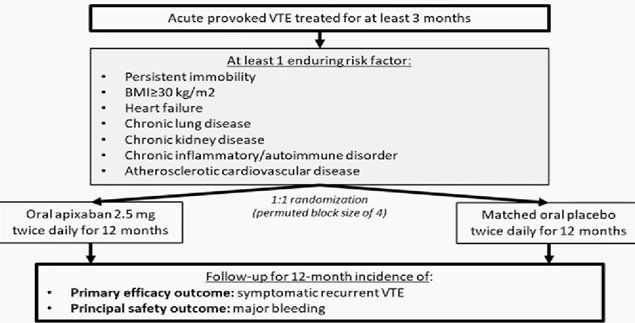

the distinction between provoked VTE—the focus of HI-PRO—and nonprovoked VTE is relevant to the duration of anticoagulant therapy that follows.
“Unprovoked venous thromboembolism is typically treated without a predetermined stop date and provoked venous thromboembolism given time-limited anticoagulation of 3 months For patients who experience VTE provoked by surgery, immobility, or other factors and who face ongoing risk factors, 12 months of low-intensity apixaban therapy reduces the odds of recurrent VTE by nearly 90%,
Piazza G, Bikdeli B, Pandey AK, et al. Apixaban for extended treatment of provoked venous thromboembolism. N Engl J Med. 2025;Epub ahead of print.
Zakai NA. Art within evidence — balancing anticoagulant duration in VTE care. NEngl J Med 2025 in press
Eμβόλια και Καρδιαγγειακά καταιγισμός δημοσιευσεων
The importance of vaccination as a cardioprotective effort has been highlighted recently by the European Society of Cardiology, which issued a consensus statement in June calling for routine vaccination to become the fourth major pillar of cardiovascular prevention alongside antihypertensives, lipid-lowering drugs, and medications that treat diabetes. That was followed this month by a recommendation from the American College of Cardiology that adults with heart disease should get vaccinated against a range of respiratory viruses, including influenza, COVID, and RSV, and other diseases where vaccination is shown to offer cardiovascular protective benefits. Heidenreich PA, et al. J Am Coll Cardiol. 2025;doi:10.1016/j.jacc.2025.07.003.
Kia sto ESC είχαμε 3 RCTs The results of the flu DANFLU-2 and GALFLU(>65) and RSV DAN-RSV,(>60) the Danish group also found vaccination against respiratory syncytial virus (RSV trials provide more evidence to support those recommendations.
in a pooled analysis, the FLUNITY-HD study, which Biering-Sørensen also led and presented at ESC. The FLUNITY-HD data will be published in an upcoming issue of The Lancet. 2025 in press
Johansen ND, Modin D, Loiacono MM, et al. High-dose influenza vaccine effectiveness against hospitalization in older adults. N Engl J Med. Published online August 30, 2025. doi:10.1056/NEJMoa2509907.
Johansen ND, Modin D, Loiancono MM, et al. High-dose vs standard-dose influenza vaccine and cardiovascular outcomes in older adults: a prespecified secondary analysis of the DANFLU-2 randomized clinical trial. JAMA Cardiol. Published online August 30, 2025
Bhatt A et al Cardiovascular-Focused Messaging to Improve Influenza Vaccination Rates. N Engl J Med Evidence 2025 in press
HELP-MI SWEDEHEART Trial
Among unselected patients with acute MI, routine H pylori screening did not significantly reduce the risk of upper gastrointestinal bleeding JAMA 2025 in pree
